Clinical Pilot Study Confirms the Accuracy and Effectiveness of Pixee Medical's Knee+ Solution Using Vuzix M400 Smart Glasses for Total Knee Arthroplasty
- Vuzix receives follow-on M400 Smart Glasses order from Pixee Medical
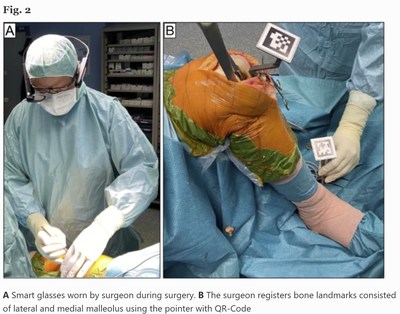
ROCHESTER, N.Y., Sept. 17, 2021 /PRNewswire/ -- Vuzix® Corporation (NASDAQ: VUZI), ("Vuzix" or, the "Company"), a leading supplier of Smart Glasses and Augmented Reality (AR) technology and products, today announced that the Vuzix M400 Smart Glasses were recently featured in a clinical pilot study that examined the accuracy of the Pixee Medical Knee + augmented reality navigation in performing Total Knee Arthroplasty (TKA). The preliminary results of the study, as discussed in the article published in the Journal of Experimental Orthopaedics, found that the Knee + system is accurate and effective to perform TKA, and that translation from pilot study to high-level prospective studies is warranted to assess accuracy and cost-effective analysis compared to conventional techniques.
Pixee Medical has now shipped more than 100 Knee+ systems, which allow the surgeon to view the tibial and femur axis superimposed on the surgical field through smart glasses. It provides real-time information during surgery and intraoperative feedback. The surgeon can choose varus/valgus angle and posterior slope on tibial cut, the valgus distal femoral cut and flexion/extension of femoral component on femoral side.
Additionally, to support the commercialization and rollout of the augmented reality navigation system across the US market, Pixee Medical placed a follow-on M400 Smart Glasses order which was delivered during the third quarter.
The full journal article can be accessed through this link: https://jeo-esska.springeropen.com/articles/10.1186/s40634-021-00374-7.
"Pixee Medical's Knee+ Total Knee Arthroplasty product is a scalable solution that continues to meet the needs of surgeons and provides a cutting-edge technology that is easy to use. We plan to expand quickly worldwide, and a next step will be to enter the US market with a focus on the growing ambulatory surgery centers (ASC) market. Our attractive AR smart glasses-based product is affordable and does not require consumable management," commented Sébastien Henry, Founding President of Pixee Medical.
"Pixee Medical continues to make great strides to support the surgical community with their innovative Knee+ AR solution, with a growing installed base in Europe and US operations just getting underway," said Paul Travers, President and Chief Executive Officer at Vuzix.
About Vuzix Corporation
Vuzix is a leading supplier of Smart Glasses and Augmented Reality (AR) technologies and products for the consumer and enterprise markets. The Company's products include personal display and wearable computing devices that offer users a portable high-quality viewing experience, provide solutions for mobility, wearable displays and augmented reality. Vuzix holds 210 patents and patents pending and numerous IP licenses in the Video Eyewear field. The Company has won Consumer Electronics Show (or CES) awards for innovation for the years 2005 to 2021 and several wireless technology innovation awards among others. Founded in 1997, Vuzix is a public company (NASDAQ: VUZI) with offices in Rochester, NY, Oxford, UK, and Tokyo, Japan. For more information, visit the Vuzix website, Twitter and Facebook pages.
Forward-Looking Statements Disclaimer
Certain statements contained in this news release are "forward-looking statements" within the meaning of the Securities Litigation Reform Act of 1995 and applicable Canadian securities laws. Forward looking statements contained in this release relate to Vuzix Smart Glasses, our business relationship and future opportunities with Pixee Medical, and among other things the Company's leadership in the Smart Glasses and AR display industry. They are generally identified by words such as "believes," "may," "expects," "anticipates," "should" and similar expressions. Readers should not place undue reliance on such forward-looking statements, which are based upon the Company's beliefs and assumptions as of the date of this release. The Company's actual results could differ materially due to risk factors and other items described in more detail in the "Risk Factors" section of the Company's Annual Reports and MD&A filed with the United States Securities and Exchange Commission and applicable Canadian securities regulators (copies of which may be obtained at www.sedar.com or www.sec.gov). Subsequent events and developments may cause these forward-looking statements to change. The Company specifically disclaims any obligation or intention to update or revise these forward-looking statements as a result of changed events or circumstances that occur after the date of this release, except as required by applicable law.
Vuzix Media and Investor Relations Contact:
Ed McGregor, Director of Investor Relations,
Vuzix Corporation
ed_mcgregor@vuzix.com
Tel: (585) 359-5985
Vuzix Corporation, 25 Hendrix Road, West Henrietta, NY 14586 USA,
Investor Information – IR@vuzix.com www.vuzix.com
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/clinical-pilot-study-confirms-the-accuracy-and-effectiveness-of-pixee-medicals-knee-solution-using-vuzix-m400-smart-glasses-for-total-knee-arthroplasty-301379539.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/clinical-pilot-study-confirms-the-accuracy-and-effectiveness-of-pixee-medicals-knee-solution-using-vuzix-m400-smart-glasses-for-total-knee-arthroplasty-301379539.html
SOURCE Vuzix Corporation
Released September 17, 2021